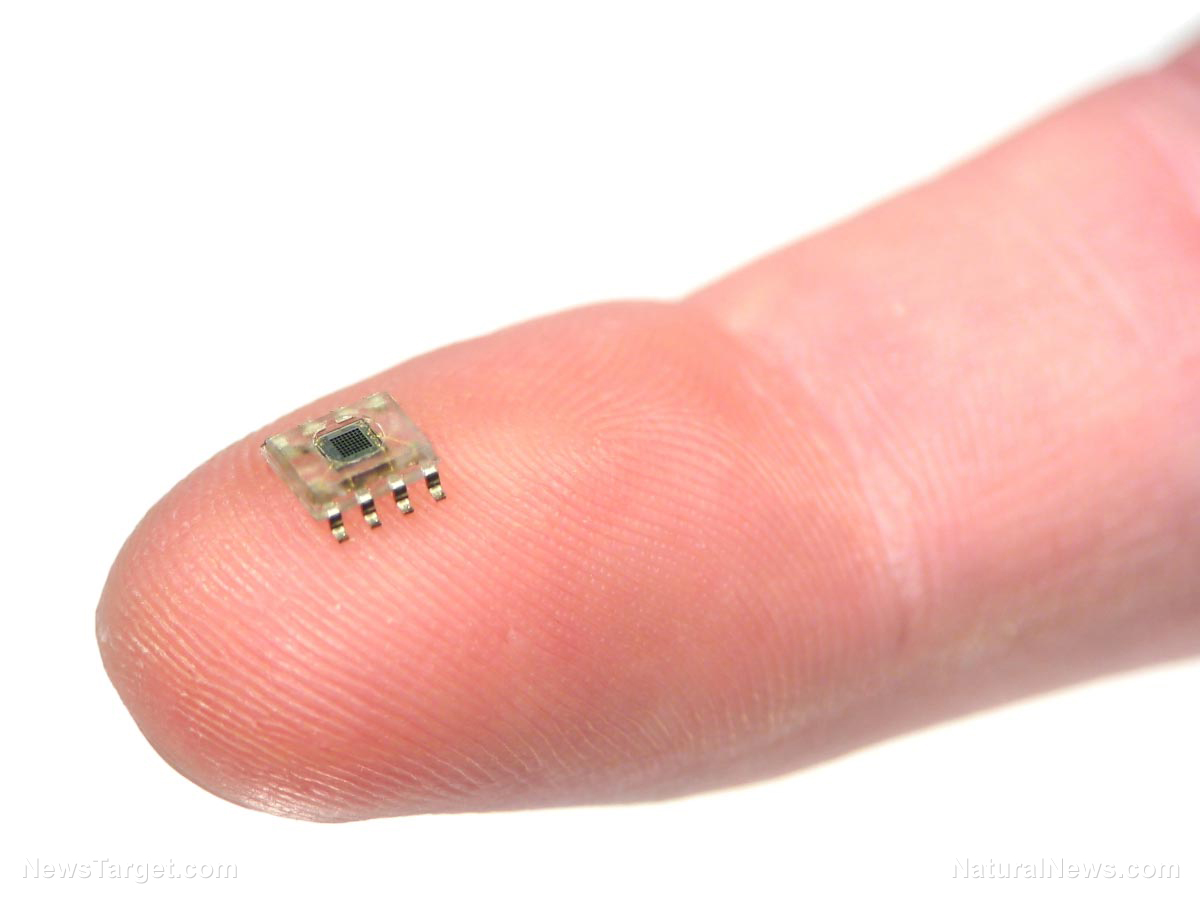
Elon Musk’s Neuralink receives FDA approval to begin clinical trials for brain implants
06/01/2023 / By Ramon Tomey
Elon Musk’s Neuralink has received approval from the Food and Drug Administration (FDA) to begin clinical trials for its brain implants.
According to Ars Technica‘s John Timmer, Musk gave an update regarding Neuralink back in December 2022. He said that time that Neuralink was close to starting clinical trials of its flagship brain implants, albeit still six months away. Timmer noted that Musk has been claiming that trials would start soon as early as 2020.
“This time, it seems that the company was correct,” he said, citing a May 26 tweet from the company. “Typically, when the FDA rejects an application for clinical trials, it is willing to communicate in detail why it found the plan for trials insufficient.”
Neuralink tweeted: “We are excited to share that we have received the FDA’s approval to launch our first in-human clinical study. Recruitment is not yet open for our clinical trial, [however]. We’ll announce more information on this soon.”
“This is the result of incredible work by the Neuralink team in close collaboration with the FDA, and represents an important first step that will one day allow our technology to help many people.”
However, Timmer noted that the trial is yet to get off the ground. He wrote: “Neuralink is not ready to start recruiting test subjects, and there are no details about what the trials will entail. Searching the ClinicalTrials.gov database for ‘Neuralink’ also turns up nothing.”
“Typically, the initial trials are small and focused entirely on safety and effectiveness. Given that Neuralink is developing both brain implants and a surgical robot to do the implanting, there will be a lot that needs testing. It’s likely that these will focus on the implants first given that other implants have already been tested in humans, whereas an equivalent surgical robot has not.”
Despite the snags, Timmer described the update as “a positive sign for Neuralink that the company was able to address the concerns of federal regulators in a relatively short period.”
FDA earlier rejected Neuralink’s plan over SAFETY concerns
Back in March, the FDA rejected Neuralink’s proposal to test implants in humans. The regulator outlined dozens of issues the company must address before it can proceed with clinical trials. Seven current and former FDA employees confirmed the agency’s decision to Reuters. (Related: FDA rejects Elon Musk’s bid to test Neuralink brain implants in humans.)
Some of the issues the FDA touched on involved the implant’s lithium battery, and the potential for its tiny wires to migrate to other areas of the brain. Questions over whether the device can be removed and how can it be done so without damaging brain tissue were also put forward.
According to one of the seven sources, Musk operates Neuralink in the same way as electric vehicle company Tesla. The latter, which is also owned by the South African-born tech mogul, brought several electric cars to market relatively quickly.
“He can’t appreciate that this is not a car,” the source said. “This is a person’s brain. This is not a toy.”
Owen Faris, principal deputy director of the FDA’s Office of Product Evaluation and Quality, emphasized the agency’s high standards in vetting all products even as it aims to expedite reviews. He said: “Innovation and safety are not an either-or scenario.”
Kip Ludwig, former program director of neural engineering at the National Institutes of Health, remarked that Musk’s claims and impatience pose a critical test for the FDA in balancing demands for speedy review with the diligence that safety and efficacy call for.
“Everybody in the industry was saying: ‘Oh my God, they’re going to run straight into a brick wall,'” Ludwig said of Musk’s application. “Neuralink doesn’t appear to have the mindset and experience that’s needed to get this to market anytime soon.”
ElonMuskWatch.com has more stories about Neuralink.
Watch this video that discusses the FDA’s approval of clinical trials for Neuralink brain implants in humans.
This video is from the Thrivetime Show channel on Brighteon.com.
More related stories:
FDA greenlights first clinical trials of Elon Musk’s human brain implants.
Neuralink brain implants are ready for injection into humans, says Elon Musk.
FDA approves Elon Musk’s Neuralink brain implants that seek to CONTROL the human mind.
Ethical questions raised after Elon Musk’s Neuralink company implants chip in monkey’s brain.
Sources include:
Submit a correction >>
Tagged Under:
big government, biotech, brain health, brain implants, Clinical trials, computing, corporations, Dangerous, Elon Musk, FDA, future science, future tech, Glitch, health science, human trials, inventions, medical experiments, Neuralink, research, safety concerns, transhumanism
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 COMPUTING NEWS